Why might the genetic mutations leading to hereditary hemochromatosis have offered an evolutionarily advantage to our Neolithic ancestors?
A great paradox of medical genetics is that certain deadly inherited illnesses have persisted across generations because they may actually protect carriers from other deadly illnesses. The cystic fibrosis gene helps defend the lungs against tuberculosis, while sickle cell trait can help the blood eliminate malaria. While not every genetic disorder follows this pattern, there are more than one may think. Hereditary hemochromatosis (HH), in which the body becomes overloaded with iron, is such a disease. To explore the reasons why, we must go on a fascinating journey through not only the human body, but the history of human civilization itself.
Let’s talk about the genetics and pathophysiology of HH first. Hereditary Hemochromatosis literally means “blood pigment color,” as early pathologists assumed the darkening they saw inside organs was from excessive internal bleeding. HH commonly involves a mutation of the HFE gene, which encodes for the human homeostatic iron regulator protein. As the name suggests, this protein helps regulate iron absorption by binding to certain transferrin receptors (TFR) of hepatic cells and leukocytes such as tissue neutrophils and monocytes. In the liver, this HFE-TFR complex binds transferrin, the protein that carries iron through the blood. The binding controls production of hepcidin, another regulatory protein which acts to suppress absorption of iron in the gut (as transferrin increases, more hepcidin is produced to help balance iron levels).

The precise mechanism of how HFE and the TFR interact and regulate hepcidin is unclear, but the end result is that patients with mutated HFE do not produce enough hepcidin. This leads to unchecked iron absorption, and deposition of iron in various organs such as the heart, liver, thyroid, and even the skin and joints. Because there are so many places the excess iron can accumulate, HH has a variety of presentations, from cirrhosis to heart failure to early-onset arthritis and skin darkening.

The two main gene variants of HFE that cause HH are H63D and C282Y. In one study of 100,000 patients in Canada with iron overload, patients who were homozygous for C282Y had the worst iron overload, and that genotype was most prevalent in white patients. Although HFE mutations are the most common source of HH, other genes can be involved, such as HJV, which is more prevalent in South Asia and causes juvenile HH. Nor is HH the only inherited cause of iron overload – ferroportin disease, which is most common in Africa, causes excess iron storage just in the liver, leading to the unusual clinical scenario of iron-overload with anemia.
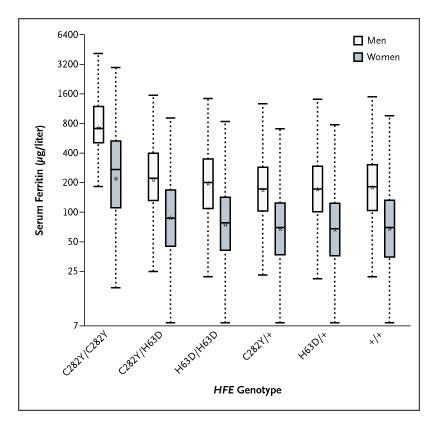
The clinical course of at-risk patients depends on which gene mutations they carry and in which combination. One functioning HFE gene is usually enough to prevent symptoms. Patients who are homozygous (with two copies) for a mutation often present in their 40’s-50’s, but that presentation may be delayed in patients who menstruate, as they have spent years with regular blood, and therefore iron, loss. Patients with compound heterozygosity, meaning they have one H63D allele and one C282Y allele, are also less likely to develop iron overload. Patients with some less-common non-HFE mutations are also at increased risk of iron overload.
HH is clearly not a disease one would wish on anyone. So how have these gene variants survived across generations? As always, there are several theories. The first involves the dietary changes of the Neolithic revolution. Approximately 10,000 years ago in Mesopotamia, humans began to transition from hunter-gatherer lifestyles to dedicated agriculture, and over several thousand years these changes spread to Central and Northern Europe. In these cold climates, the diet became reliant on hardy cereal grains and milk from livestock. Inhabitants even evolved to produce lactase after infancy so they could keep drinking milk as adults. However, this was not an iron-friendly diet. Unlike their meat, the milk of cows is a poor source of iron, which is why babies who only get cow’s milk instead of formula can get iron-deficient. Cereal grains bind iron on their surfaces via phytates, a stored form of phosphorus, which prevents iron absorption. It makes sense then that the most common HFE mutations appear to have arisen in Northern Europe around 4000 B.C., as less HFE could have meant more iron absorption, and that people who now identify as white may be the descendants of these iron-poor northerners.

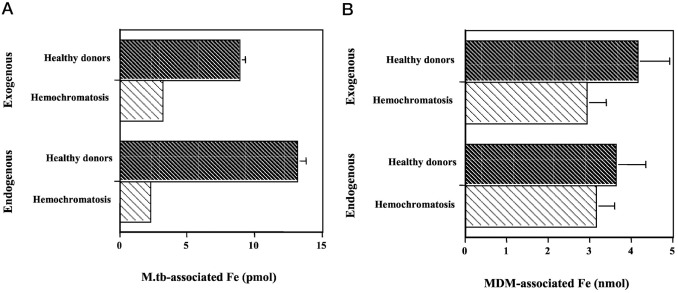
The milk-and-cereal hypothesis may not be the full story, though. As diets diversified across centuries, the HFE mutations only increased in prevalence and distribution, with prevalence of C282Y peaking at 10% in the Middle Ages ranging from Western Europe to Asia. There must have been some other environmental pressure to encourage the proliferation of these mutations. Although HH is a disease of iron overload, the dysfunctional HFE-TFR complex prevents monocytes, macrophages, and neutrophils from absorbing iron. One of the most deadly bacteria in history, Mycobacterium tuberculosis (MTB), invades macrophages and grows via their stored iron. One study showed that in the macrophages of HH patients, MTB was able to absorb significantly less iron. As tuberculosis, particularly scrofula (in which the bacteria infects the neck lymph nodes), began spreading during the Middle Ages, one sees that variants of the HFE and cystic fibrosis genes that protect against MTB began to thrive, and variants which confer susceptibility began to decline.
Some more recent research indicates that HFE mutations may affect other immune functions. HFE is able to bind to TFR by lodging in the cell membrane. Its shape (three alpha-domain proteins bound to a B2 microglobulin light chain) is extremely similar to the major histocompatibility complex 1 (MHC1), a protein which presents self-antigens from the cell to cytotoxic CD8+ T-Cells. Some studies of the C282Y variant show it can affect MHC1 expression, T-Cell activation and inflammatory cytokine expression. However, much of this is theoretical, as it is unclear if HFE mutations actually help deter viral infections, and even if so, if there ever was a strong enough evolutionary pressure from viruses to select for them.

HFE mutations may even cause certain diseases to proliferate. Like MTB, Yersinia, the bacteria which causes bubonic plague, also loves iron, but grows best in blood rather than inside macrophages. The bloodstream of someone with HH is a fertile environment for Yersinia and other siderophilic (iron-utilizing) bacteria, making HH a risk factor for serious bloodstream infections. The devastation of the Black Death in mid 14th-century Europe may have caused HFE mutation frequency to drop to modern levels after the rise in response to MTB, but there is no clear proof.
The protective effects of HFE mutations are more important historically than currently. However, that doesn’t mean they can’t bring any benefit. Even if one is a heterozygote and is spared of HH, one still has increased iron circulation compared to the general population, which may increase the oxygen-carrying capacity of the blood. This is not a speculation – in one study of high-level French athletes in anaerobic sports like judo, rowing and nordic skiing, 80% of them had heterozygous HFE mutations.
Take Home Points
- Hereditary hemochromatosis is most commonly caused by variants in the HFE gene, which affect the regulation of iron uptake in the bowel and in immune cells.
- HFE gene variants may have had beneficial advantage for avoiding iron deficiency during the shift to agrarian lifestyles and consumption of iron-chelating cereal grains in Europe in the Neolithic age.
- A second selective pressure to favor these HFE gene variants may have occurred due to protection from mycobacterium tuberculosis and salmonella typhi in the Middle Ages.
- There are likely additional ways that HFE modulates the immune system that we’re still learning about
Listen to the episode
https://directory.libsyn.com/episode/index/id/30053588
Credits & Citation
◾️Episode written by Hannah Abrams
◾️ Show notes written by Giancarlo Buonomo and Hannah Abrams
◾️Audio edited by Clair Morgan of nodderly.com
Cooper AZ, Breu AC, Abrams HR. History & Hemochromatosis: The Ins and Outs of Iron. The Curious Clinicians Podcast. February 21st, 2024.
This episode is sponsored by Audible! New members can try Audible free for 30 days using our own Curious Clinicians podcast code – visit Audible.com/TCCPOD or text TCCPOD to 500-500.


Very interesting and well done podcast on hemochromatosis. Perhaps worth noting that the inly way for the body to remove iron is via bleeding.
I would note that this is one of the reversible causes of LV systolic dysfunction that is easily diagnosed and treated and should be part of the workup for unexplained CHF.
It would be fun to note that the primary treatment, phlebotomy, is one of the very few medical treatments that we still use that date back hundreds of years.
Thanks
Steve B breecker, M.D. Cardiology
LikeLike