For this episode of the podcast, Avi and the team discussed “normal” saline and why it isn’t so normal. This was based largely on the tweetorial he posted on New Year’s Eve, 2019.
A Little History
Saline was originally used in the 1830s by a Scottish physician named Thomas Latta to treat cholera patients during an epidemic that affected most of Europe. His solution contained the following:
- Sodium 134 mmol/l
- Chloride 118 mmol/l)
- Bicarbonate 16 mmol/l
This is very different than the components of contemporary “normal” 0.9% saline:
- Sodium 154 mosm/l
- Chloride 154 mmol/l
This solution (0.9% saline*) was developed by Jakob Hamburger, a Dutch chemist, based on an erroneous assessment of the composition of mammalian blood. He called it “normal” which probably helped it gain widespread use, even though it is definitely not normal.
What about Lactated Ringers?
Sydney Ringer invented his famous solution in the 1880s. This was a time when intravenous fluids (IVFs) were a drug du jour and new ones were popping up all the time. Ringer’s solution contained sodium, chloride, potassium, bicarbonate, and calcium, titrated to allow an explanted frog heart to continue beating.
In the 1930s a pediatrician named Alexis Hartmann added lactate as another source of bicarbonate buffer (lactate is metabolized to bicarbonate in the liver). This is the lactated ringers we continue to use today.
There are a number of differences between normal saline and lactated ringers (LR):
- LR has a pH of 6.5 versus 5.5 for normal saline .
- LR has an osmolarity of 275 mmol/L, compared with 308 for normal saline.
- LR has significantly less chloride than saline, 109 mmol/L vs 154 mmol/L
Why are these differences problematic?
Multiple studies have shown an association between saline use and acute kidney injury. One key trial was Saline against Lactated Ringer’s or Plasma-Lyte in the Emergency Department (aka SALT-ED) trial.
SALT-ED included more than 13,000 emergency department patients who required intravenous fluids and who did NOT end up in the intensive care unit. They were randomized to receive either a balanced fluid (e.g., LR or PlasmaLyte) or saline.
The primary outcome was hospital-free days (days alive after discharge before day 28). Secondary outcomes included major adverse kidney (MAKE) events within 30 days. Such adverse events included:
• Death from any cause
• New renal-replacement therapy
• Persistent renal dysfunction
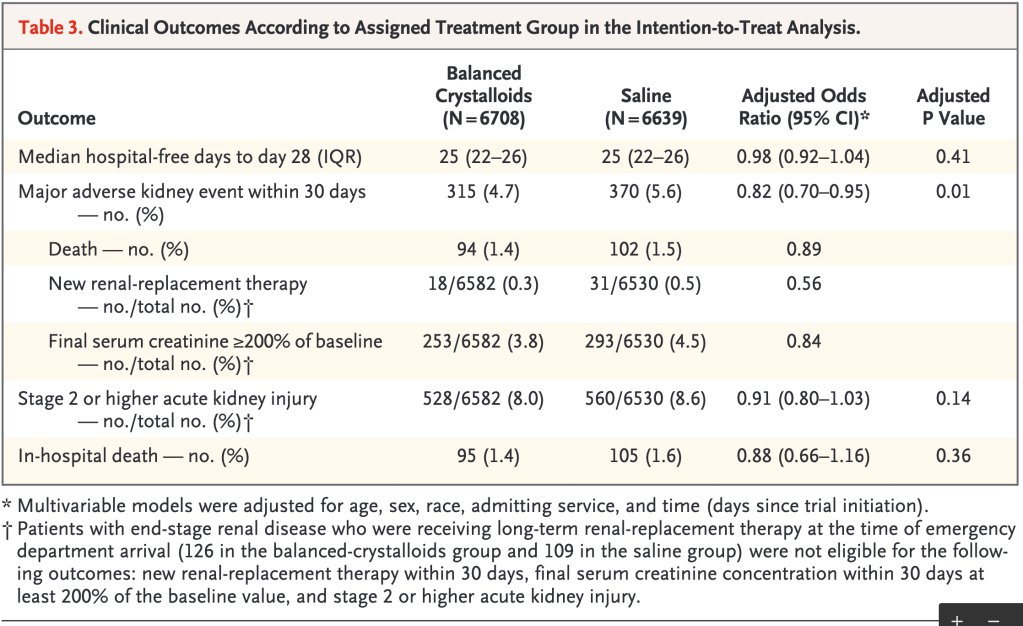
As you can see in Table 3 below, the rate of MAKE was lower in the balanced crystalloid group (4.7% versus 5.6%).
How does saline affect the kidney?
The difference in the rate of adverse renal outcomes seen in the SALT-ED trial and others may relate to the difference in chloride concentration between fluids: LR and PlasmaLyte both have a chloride concentration of about 100 mmol/L, while saline is far higher (154 mmol/L).
It turns out that chloride can decrease renal blood blow (RBF) and glomerular filtration rate (GFR). This was first studied in dogs and reported in 1983. Infusing high chloride-containing fluids decreased the dogs’ RBF and GFR anywhere from 10-60 percent.
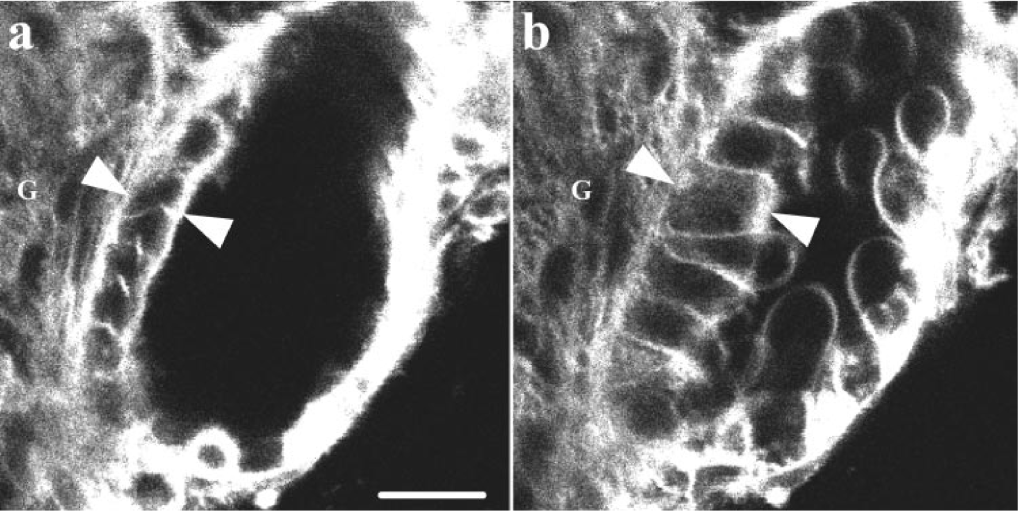
The mechanism of decreased RBF relates to the macula densa. The macula densa resides in the distal convoluted tubule (DCT) and is immediately adjacent to the afferent glomerular arterioles. It senses changes in sodium and chloride levels in the DCT and responds by modulating renal blood flow and GFR. With saline administration, high chloride levels are delivered to the macula densa, leading the cells to swell (see picture).
Ion channels then open, resulting in the release of ATP. ATP diffuses to the afferent arterioles, causing vasoconstriction, thereby reducing in renal blood flow and glomerular filtration rate.
This process is called tubuloglomerular feedback and it acts as a form of autoregulation for the kidney to control its own perfusion. If the macula densa receives a high chloride load, this is a signal to reduce GFR to reduce chloride filtration.
Take home points
- “Normal” saline is not normal. It isn’t even even physiologic!
- There is evidence of increased renal injury with the use of saline compared to balanced IV fluids (e.g., lactated ringers).
- The higher chloride levels found in saline causes afferent renal arteriole vasoconstriction via signalling from the macula densa, reducing renal blood flow and GFR.
Learning Objectives
- Understand the potential effects of saline solution on renal function, in comparison with other crystalloid solutions such as Lactated Ringer’s
- Understand the differences in chloride content between different crystalloid solutions
- Learn the mechanism by which saline and other high chloride-containing solutions can decrease renal perfusion
CME/MOC
We are excited that The Curious Clinicians have partnered with VCU Health Continuing Education to offer continuing education credits for physicians and other healthcare professionals. Visit VCU Health for more information.
Listen to the episode
https://curiousclinicians.libsyn.com/episode-7-abnormal-saline
Credits & Citation
◾️Episode written by Avi Cooper
◾️Episode audio edited by Clair Morgan of Nodderly
◾️Show notes by Tony Breu and Avi Cooper
Cooper AZ, Abrams HR, Breu AC. Is saline nephrotoxic? The Curious Clinicians Podcast. August 19, 2020. https://curiousclinicians.com/2020/08/19/episode-7-is-saline-nephrotoxic/
Opening image source: https://www.cbsnews.com/news/doctors-sound-the-alarm-on-possible-harms-of-saline-in-iv-bags/


Excellent explanation
LikeLike