Why don’t we transfuse to a normal hemoglobin?
Patients are so much more than numbers on a chart, but those numbers really are important. Lab values like potassium and white blood cells are not just figures to be balanced like there’s an impending IRS audit – they give quantifiable information on how a patient is doing. It follows, then, that getting a patient’s lab values to a normal range is part of getting them back to health. For example, when repleting potassium, we want to get it back to 4 (the normal range is 3.5-5 mmol/L). There is one value, however, which we not only leave alone until it is well below normal, but only replenish to barely half the normal value: Hemoglobin (Hgb). We’d never let a patient leave the office with a potassium of 10, or discontinue antibiotics with a white count of “only” 18,000. So why do we replenish Hgb only to >7 g/dL when normal is around 14 g/dL?
Transfusing human blood and blood products is, in and of itself, a major achievement in medical history. For centuries, bloodletting was considered a cure for a variety of ailments. As physicians in the 1600’s began understanding the dangers of severe blood loss, they experimented with red wine (unsurprisingly, this proved unsuccessful). In 1667, the French physician Jean-Baptiste Denise transfused a small amount of lambs blood into a 15 year-old boy who had been, ironically, over-bled by his doctor. Finally in 1818, the first recorded instance of human-human transfusion took place in London when James Blundfield and Henry Crane transfused approximately 400mL of blood from several different donors into a man bleeding to death from an apparent stomach cancer. Reading Blundfield’s account, one recognizes the classic signs of hemorrhagic shock: “The pulse was small, and feeble … the temperature of the limbs was falling, and the mind sinking into a state of insensibility.” As opposed to wine or animal blood transfusions, no apparent anaphylactic or hemolytic reaction occurred, and afterwards Blundfield noted the patient had improved color and spoke “in a more audible whisper than he had made use of before.”
Mind you, this took place over 80 years before the discovery of something we think of as fundamental to transfusion: Blood types. After Karl Landstein’s demonstration of the different blood groups in 1900, further innovations in separating blood into components, storing them, and safely transfusing quickly followed. The low threshold, therefore, does not seem to have been born from a longstanding desire to avoid transfusion due to technical or safety concerns. Nor does it stem solely from blood shortages, although those certainly are still an issue. Instead, multiple studies indicate that a conservative approach to transfusion is not just acceptable, but may even be preferable.

In 1999, the TRICC ( Transfusion Requirements in Critical Care) trial was published in The New England Journal of Medicine. This study took 838 critically-ill patients and randomized them to either a liberal (target Hgb 10-12 g/dL) or conservative ( 7-9 d/gL) transfusion strategy. Although the 30-day mortality rates between the groups were the same, the conservative group had lower in-hospital mortality and fewer cardiac complications such as pulmonary edema and myocardial infarction. Since then, more than 30 trials with various patient populations have been published, with nearly all supporting a conservative rather than liberal strategy. It is worth noting that none of these trials even transfused back to a “normal” hemoglobin given the well-known complications of transfusion such as volume overload and lung injury.
So how can the human body tolerate such low hemoglobin? There are a few mechanisms. The most fundamental is the hemoglobin reserve. Animal studies have shown that resting oxygen delivery to tissues is 20-30 mL O₂·kg⁻¹·min⁻¹, while the resting oxygen consumption is 6 mL O₂·kg⁻¹·min⁻¹. In other words, the blood is carrying 3-5x more oxygen than is actually utilized. This changes based on the organ (the kidneys only use ~10% of their delivered oxygen, while the hardworking heart uses over 50%), and during exercise. Overall, the large reserve of oxygen supply is protection during fluctuations in oxygen needs, perfusion (such as minor bleeding or dehydration), and oxygen levels of the blood.
Let’s focus on that last point. Oxygen delivery to tissues depends on two things: Enough oxygen carried by red blood cells (RBCs), and then enough intravascular volume to effectively carry those RBCs around the body. A patient with hemorrhagic shock has problems with both. What happens though, if someone just has the first problem? One well-known study bled volunteers to a hemoglobin of 5 g/dL, while keeping them isovolemic with albumin or plasma infusions. The subjects (who were at rest), had some predictable physiologic responses: Heart rate (HR), stroke volume (SV), and cardiac output (CO) all increased, improving blood delivery to tissues. The heightened CO increased oxygen consumption, but the subjects had no increase in serum lactate (a marker of anaerobic respiration). This meant that adequate oxygen delivery must have been maintained, despite the seemingly critical Hgb drop.

One unexpected response was a decrease in systemic vascular resistance (SVR). Hypovolemic patients typically have an increased SVR (via decrease in blood vessel radius) in order to maintain perfusion pressure to vital organs. According to Poiseuille’s Law, the flow of a liquid through a cylindrical tube, like a blood vessel, is directly proportional to the fourth power of the radius of the tube, the pressure gradient along the tube, and is inversely to both the proportional length of the tube and the viscosity of the fluid. The subjects’ blood was less viscous than normal, due to the isovolemic replenishment with plasma and albumin, making it flow more easily. This in turn helps increase CO, as there is more preload and less afterload to pump that increased volume against.

These experiments involved acute anemia and rapid correction of volume. What about chronic anemia? One study from 1963 took 51 patients with chronic anemia, 45 of whom were suffering from ankylostomiasis, an intestinal hookworm infection. They were separated into two groups, one with an average Hgb of 3.0 g/dL, the other with an average of 4.5 g/dL. The first group had, on average, a higher HR, SV and cardiac index (CI). However, both groups maintained their oxygen consumption, a sign that adequate oxygen was being delivered to tissues. One subject in the study, Patient One, had a starting Hgb of 1.5 g/dL, a value which could make a new intern call a code. He had a normal oxygen consumption, however, a heart rate of only 100, and a CI and SV that were 3 and 2x normal, respectively. After being transfused to a Hgb of 10 g/dL, the HR was unchanged, but the CI and SV returned to normal, which suggested that the SV and CI had been increased not as a perfusion-maintaining adaptation but rather, just like in the acute anemia study, secondary to increased flow from lower blood viscosity.

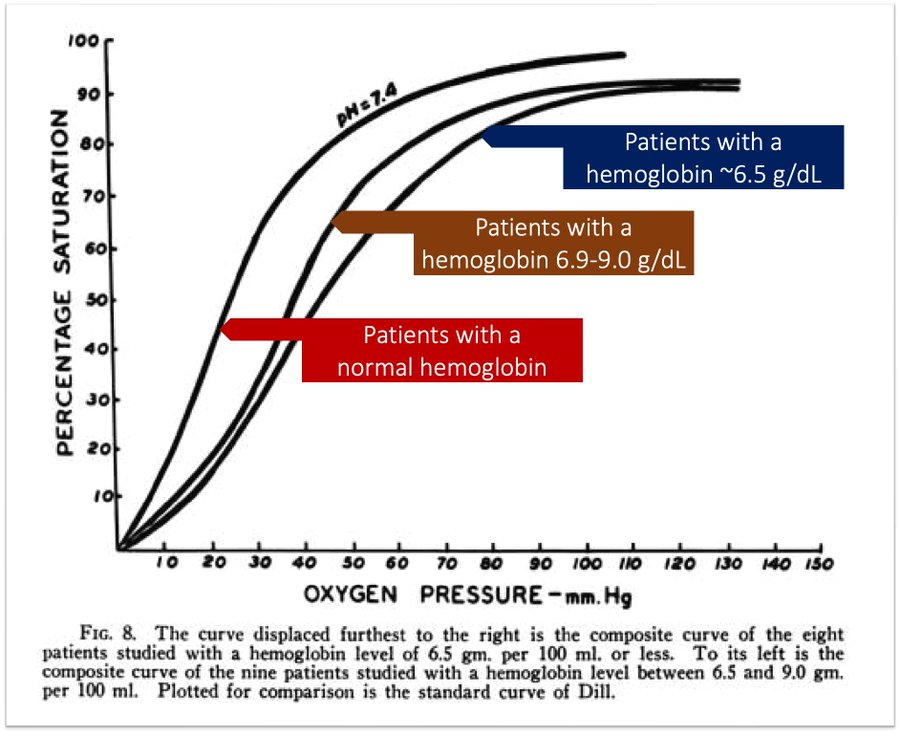
One other adaptation that these subjects had was increased oxygen delivery at the cellular level. Starting at a Hgb of around 9 g/dL and peaking after 6.5 g/dL, there is increased synthesis of 2,3-diphosphoglycerate (2,3-DPG). This compound causes a rightwards shift of the oxyhemoglobin dissociation curve, meaning that Hgb will release oxygen more readily. This process takes 12-36 hours, so it is not as applicable in cases of hyperacute anemia like hemorrhagic shock.
These adaptations aren’t perfect, of course – otherwise, there’d be no need to ever transfuse. In combination with intensive medical therapy, however, patients can survive shocking amounts of blood loss. The lowest “survived” Hgb ever recorded in a human was 1.4 g/dL, in a Jehovah’s Witness who was hemorrhaging from placenta previa but refusing blood products. By placing her on FiO2 of 100%, maintaining isovolemia, supplementing iron and sedating and cooling her to reduce O2 demand, the patient survived. Note that this was the lowest recorded in a human, though. The Antarctic Icefish happily swims around its frigid home with an Hgb of … zero. Its large heart and vessels, increased blood volume and low metabolic demands allow it to deliver the small amount of O2 dissolved in its blood around its body.
While we may be losing this cold-blooded battle to the Icefish, humans have several remarkable adaptations to low Hgb. Our large oxygen reserve and ability to unload more oxygen during anemia maintains oxygenation at a cellular level, while decreased blood viscosity and accompanying increased cardiac output keep blood flowing throughout the body (especially to vital organs like the heart and brain). As transfusion isn’t a risk-free procedure, these adaptations allow us to transfuse to levels barely half of normal.
Want to know how we got to that specific figure of >7 g/dL? Check out Tony Breu’s most recent Origin Stories post!
Take Home Points
- When transfusing, our target is not a normal hemoglobin
- This stems from risk/benefit ratio, evidence-based medicine, and blood scarcity
- Fortunately, the human body has more hemoglobin than is needed
- Many physiologic adaptations ensue in acute and chronic anemia
Link to Related Tweetorials
CME/MOC
Click here to obtain AMA PRA Category 1 Credits™ (0.5 hours), Non-Physician Attendance (0.5 hours), or ABIM MOC Part 2 (0.5 hours).
Listen to the episode
https://directory.libsyn.com/episode/index/id/29522553
Credits & Citation
◾️Episode written by Tony Breu
◾️ Show notes written by Giancarlo Buonomo and Tony Breu
◾️Audio edited by Clair Morgan of nodderly.com
Breu AC, Cooper AZ, Abrams HR. When Normal Labs Aren’t Necessary. The Curious Clinicians Podcast. December 19th, 2023.
Image credit: https://www.mindray.com/en/media-center/blogs/transfusion-safety-of-donated-blood

Awesome write-up. Looking forward to listening to the episode when I get a chance. Keep up the strong work everyone!
LikeLike