How did this anti-nausea drug cause thousands of birth defects?
On the Curious Clinicians, we like to often discuss more lighthearted topics, such as why some people think cilantro tastes like soap or whether the post-Thanksgiving turkey coma is a real thing. This episode deals with something whose pathophysiology and history are considerably darker, but important to talk about nonetheless: How thalidomide was heavily, irresponsibly marketed and prescribed to pregnant women in the 1950’s and 60’s, and ended up causing birth defects in thousands of babies across the world. Like the complex history of opioids, the tale of thalidomide is full of medical innovation, corporate hubris, public health heroism, and a current middle ground between patient safety and the very real benefits of the drug.
Developed in 1952 as a sedative and antiemetic, thalidomide was found to have minimal biological effects in early animal studies and therefore abandoned. In 1957, a drug company called Chemie Grünenthal bought the rights, performed clinical trials which showed thalidomide’s efficacy in treating morning sickness, and began distributing the drug around the world. Thalidomide was marketed mostly as an antiemetic for pregnant women, but was also proclaimed as a cure-all “Wonder Drug” for everything from colds to insomnia. The potentially teratogenic effects of thalidomide had not been well-studied before it became widely available over-the-counter, going by at least 37 different names including Contergan and Distaval. That did not stop advertisements from bold claims such as “Distaval can be given with complete safety to pregnant women and nursing mothers without adverse effect on mother or child”.

In 1961, an Australian obstetrician named William McBride published a letter in The Lancet which noted multiple instances of babies born at his Sydney hospital with congenital abnormalities, particularly underdevelopment and shortening of the femur and radius called phocomelia. Every single mother of one of these babies had taken thalidomide during pregnancy. McBride’s letter asked his fellow physicians if they too had noticed unusual birth defects that could be linked to thalidomide. The story behind McBride’s reporting about thalidomide embryopathy does have some controversy, as the initial observation about phocomelia and thalidomide may have been made by a nurse, Sister Sparrow, someone whom McBride did not credit in his letter. After publication, the Lancet letter led to thalidomide getting quickly withdrawn from the market, though at this point the drug had been used widely for 5 years, and over 10,000 children already born with limb defects.

Thalidomide had such a devastating effect because it is uniquely teratogenic, dangerous even after a single dose early in pregnancy, and was used most during the first trimester when morning sickness is often most severe. One can even predict deformities based on the day of thalidomide exposure in-utero: Days 21 and 22 would increase risk of thumb aplasia, while days 29 and 30 may affect leg development. The most common abnormalities are in the heart, eyes, ears, and most notably the long bones of the limbs, which have become something of a symbol of thalidomide toxicity.

How does thalidomide cause such devastating birth defects? There are three main theories, including altered ubiquitin ligase function, oxidative stress on the embryo, and intrinsic anti-angiogenic effects. The first theory has the strongest empirical backing. Ubiquitin is an intracellular protein that is used to “tag” other proteins for degradation, like a logger spray-painting trees to be felled. By controlling which proteins are active at which times in the cell, ubiquitin plays an essential role in cell regulation. To correctly attach to target proteins, ubiquitin must be activated, conjugated, and finally ligated to the protein via the ubiquitin-ligase complex. Thalidomide is able to bind to this complex, specifically a protein called cereblon in the ubiquitin ligase complex. The ubiquitin ligase complex plays an important role in regulating growth factor expression and therefore limb development. One 2010 chick embryo study used embryos with either normal cereblon or a thalidomide-resistant variant, and exposed both types to thalidomide. Those with normal cereblon developed limb abnormalities, while the thalidomide-resistant group had no limb abnormalities.
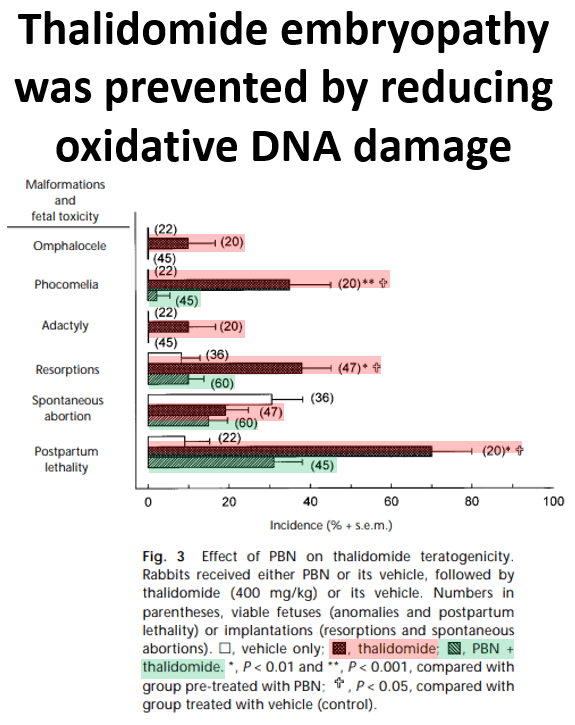
Thalidomide may also cause increased oxidative stress, i.e generation of free radicals, that damage embryonic DNA.One 1999 study of pregnant rabbits showed that thalidomide induced a 380% increase in DNA oxidation in embryos. To solidify the link between oxidative stress and thalidomide-induced abnormalities, the researchers then treated some of the rabbits with alpha-phenyl-N-t-butylnitrone (PNB), a free-radical trapping agent. Rabbits treated with PNB in-utero had almost no instances of phocomelia or other defects. Rabbits which had been exposed to thalidomide and not treated with PNB had a 35% incidence of phocomelia.
The last proposed mechanism involves disruption of angiogenesis, or growth of new blood vessels. One 2019 study showed that thalidomide reduced expression of angiogenin (a major angiogenic factor) by almost 3-fold in mice. It is no surprise, then, that some of the current uses of thalidomide are to treat epistaxis in hereditary hemorrhagic telangiectasia (HHT) and bleeding in small-bowel angiodysplasia, as both diseases involve disordered blood-vessel growth.

Returning to how thalidomide was approved for use in humans in the 1950s, it out turns that, for unknown reasons, rats and other rodents seem to be resistant to thalidomide-induced congenital defects. It also happens that thalidomide was exclusively tested for toxicity on rats. Coupled with a mistaken belief that thalidomide couldn’t cross the placenta, the pharmaceutical industry and many physicians took this as evidence that thalidomide would not be harmful to humans. In 1962, a year after McBride’s Lancet letter led numerous other clinicians to link thalidomide and birth defects, the founder of the Teratology Society was still casting doubt on thalidomide’s potential for harm. Eventually, the evidence became so overwhelming that the pharmaceutical industry developed a new standard for testing drugs: They must be tested in at least two different species, one of which cannot be a rodent. Governments also passed stricter laws about marketing claims and disclosing potential drug side effects. Although it was on the market for only a few years, thalidomide ended up changing the way drug safety is regulated.
Most of these regulations were passed in the mid-to-late 1960’s, and William McBride’s Lancet letter was published in 1961, several years after thalidomide was first sold. How is it that American children emerged largely unharmed? Sometimes, stories really do have a hero, and her name was Dr. Frances Kelsey. In 1960, the American pharmaceutical company Richardson-Merrell submitted thalidomide, licensed from Grünenthal, for approval by the FDA. The hope was to introduce it to the American market after it was such a success elsewhere. The application was reviewed by a recently-hired, Canadian-American physician and pharmacologist named Frances Kelsey. Reading over the application, Kelsey became skeptical that the provided data was sufficient to claim that thalidomide was safe. She dug deeper, and discovered new studies that linked thalidomide with peripheral neuropathy. Despite pressure from Grünenthal, she refused to approve thalidomide unless further studies were conducted. Thalidomide was never distributed widely in America, and Kelsey was awarded the President’s Award for Distinguished Federal Civilian Service, only the second woman to ever receive it at that point.

Kelsey’s steadfast opposition to thalidomide demonstrates how much power individuals can have when it comes to patient safety, especially in a process as vulnerable to financial interests as drug regulation.
Take Home Points
- >10k babies experienced thalidomide embryopathy in the 1950s-60s, most prominently with characteristic limb development disruption leading to phocomelia, though there were many other manifestations as well.
- The main proposed mechanisms include:
- Disrupted ubiquitin ligase function, which effects growth factor expression
- Oxidative stress
- Anti-angiogenic effects
- Thalidomide’s anti-angiogenic effects have clinical relevance today, with use in prevention of bleeding from disorders such as hereditary hemorrhagic telangiectasia and small-intestinal angiodysplasia, as well as multiple myeloma
- Finally, the missteps in preclinical testing and regulatory oversight that allowed thalidomide to be used in pregnancy led to a new, safer era in pharmaceutical drug development.
We also want to plug Tony’s new Substack called Origin Stories. If you like the types of questions we answer on The Curious Clinicians, you’ll definitely want to check that out and subscribe. You can find Origin Stories at tonybreu.substack.com.
Link to Related Tweetorial
CME/MOC
Click here to obtain AMA PRA Category 1 Credits™ (0.5 hours), Non-Physician Attendance (0.5 hours), or ABIM MOC Part 2 (0.5 hours).
Listen to the episode
https://directory.libsyn.com/episode/index/id/29737018
Credits & Citation
◾️Episode written by Avi Cooper
◾️ Show notes written by Giancarlo Buonomo and Avi Cooper
◾️Audio edited by Clair Morgan of nodderly.com
Cooper AZ, Breu AC, Abrams HR. Thalidomide Embryopathy. The Curious Clinicians Podcast. January 31st, 2024.
Image Credit: Science Museum Group

